The coronavirus illness 2019 (COVID-19) pandemic, which is attributable to extreme acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has claimed greater than 5.8 million lives worldwide. A number of COVID-19 vaccines have acquired emergency use authorization (EUA) from regulatory businesses all over the world, which has allowed virtually 62% of the worldwide inhabitants to be vaccinated towards SARS-CoV-2.
Earlier research have revealed that vaccination has considerably lowered mortality, extreme an infection, and even transmission of SARS-CoV-2. A few of the COVID-19 vaccines that acquired are EUA® (BioNTech/Pfizer), Vaxzevria® (AstraZeneca), and Spikevax® (Moderna) vaccines.
Research: Allergological Research in Sufferers Vaccinated Towards COVID-19 with Suspected Allergic Reactions. Picture Credit score: Microgen / Shutterstock.com
Background
The tempo of COVID-19 vaccination has suffered because of vaccine hesitancy and vaccine provide shortages. Vaccine hesitancy is pushed by varied causes, akin to worry of doable allergic reactions and negationism.
A few of the hostile results following COVID-19 vaccination have occurred as a result of activation of protecting immune responses; nevertheless, these are usually not allergic reactions. Fast hypersensitivity reactions have been attributed to the excipients polyethylene glycol (PEG) and/or polysorbate 80 (PS80), each of that are utilized in most vaccines.
It should be famous that the reactions to those compounds are very uncommon. Diagnoses of hypersensitivity to those polymers stays tough, as pores and skin exams are usually not but standardized and their efficacy isn’t properly established.
Earlier research have proven that instances of fast or delayed hypersensitivity to SARS-CoV-2 vaccines are very uncommon. Moreover, the etiopathogenic mechanism of allergic reactions to those vaccines stays unclear. Analysis has proven that anaphylaxis with COVID-19 vaccines is round 7.9 instances per million doses worldwide.
A brand new research that’s at the moment posted to the Analysis Sq.* preprint server whereas into consideration for publication in BMC One Well being Outlook, analyzes suspected reactions in sufferers vaccinated with the primary and/or second dose of SARS-CoV-2 vaccines. Herein, the researchers additionally explored the reason for these reactions amongst their parts and evaluated whether or not such reactions interfered with the vaccination protocol.
Concerning the research
Within the present research, researchers carried out a cross-sectional and descriptive research on sufferers who have been suspected to display hypersensitivity to SARS-CoV-2 vaccines. The individuals have been subjected to a pores and skin prick check (SPT) and/or intradermal check (IDT) with the vaccines and their excipients.
Histopathological and immunohistochemical research have been carried out by pores and skin biopsy in instances the place sufferers have been optimistic IDT with a specific vaccine. Lastly, scientists additionally carried out basophil activation exams (BAT) and lymphoblastic transformation exams (LTT).
Research findings
A complete of sixteen sufferers have been suspected to point out hypersensitivity to the SARS-CoV-2 vaccine. Of those, twelve sufferers acquired Comirnaty®, three acquired Vaxzevria®, and one acquired Spikevax®.
Eight sufferers had fast hypersensitivity reactions, whereas the remaining confirmed delayed reactions. The SPTs to excipients and vaccines have been destructive in all instances. Additional, IDTs with all excipients have been destructive.
In two sufferers who have been chosen optimistic IDT with the vaccine, histological and immunohistochemical research confirmed T-lymphocyte involvement. In each instances, BAT and LTT have been destructive.
In 44% of the sufferers studied, the vaccination protocol could possibly be accomplished. The remaining 56% didn’t obtain the second dose, both as a result of they refused to be vaccinated or as a result of they already had the entire routine.
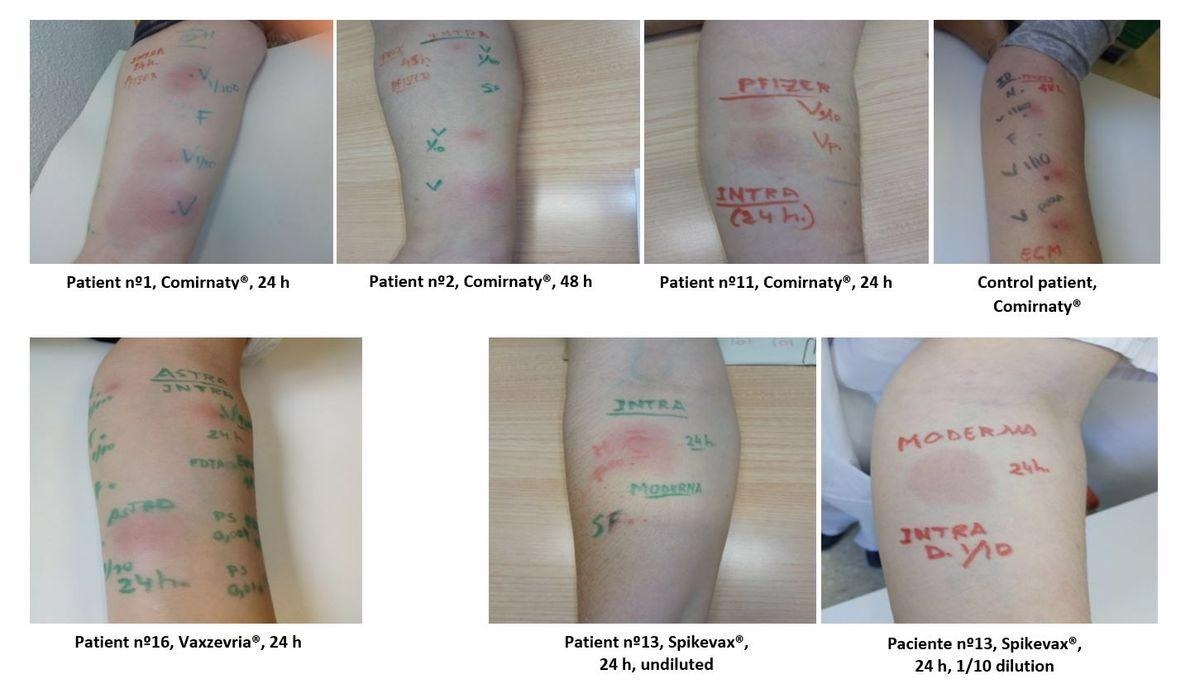
IDT with Comirnaty®, Vaxzevria® and Spikevax® For SF: management with saline resolution; Vp: undiluted vaccine; V1/100: 1/100 diluted vaccine; V1/10: 1/10 diluted vaccine
Strengths and limitations
The researchers said that the necessary medical thought is the principle power of this research. The target of analyzing the influence of an interventional screening program in high-risk sufferers is extraordinarily necessary to information public well being insurance policies.
Nevertheless, the outcomes ought to be interpreted with warning due to the small pattern dimension and the truth that all individuals got here from a really particular area. To achieve a greater understanding of the problem, research with bigger pattern sizes and higher illustration of all doable areas are wanted.
conclusions
The vaccination protocol could possibly be efficiently accomplished in roughly half of the sufferers who demonstrated doable hypersensitivity reactions to SARS-CoV-2 vaccines. That is largely due to this allergological and immunohistochemical research and is, due to this fact, a big achievement.
Sooner or later, IDTs with vaccines could possibly be a probably priceless methodology for assessing the immunogenicity of vaccines.
*Essential discover
Analysis Sq. publishes preliminary scientific reviews that aren’t peer-reviewed and, due to this fact, shouldn’t be thought to be conclusive, information medical apply/health-related conduct, or handled as established data.
Journal reference:
- Cerda, JV, Pacheco, RR, Witek, JD, et al. (2022) Allergological Research in Sufferers Vaccinated Towards COVID-19 with Suspected Allergic Reactions. Analysis Sq.. doi:10.21203/rs.3.rs-1301307/v1.

