Tumor-associated microbiota is a vital part of the tumor microenvironment (TME) throughout 33 varieties of human most cancers. Nonetheless, little proof is offered concerning the spatial distribution and localization of those microbes in tumor cells.
Addressing this hole in analysis, a latest Nature journal research evaluated spatial, mobile, and molecular host-microbe interactions in oral squamous cell carcinoma (OSCC) and colorectal most cancers (CRC). On this research, scientists mapped host–bacterial mobile, spatial, and molecular interactions throughout the TME utilizing single-cell RNA sequencing (scRNA-seq) and in situ spatial-profiling applied sciences.
Examine: Impact of the intratumoral microbiota on spatial and mobile heterogeneity in most cancers. Picture Credit score: jovan vitanovski / Shutterstock
Background
Sometimes, most cancers sufferers’ tumors comprise malignant cells surrounded by a compound community of non-malignant cells. These cells would possibly exhibit pro- or anti-tumorigenic results based mostly on their abundance and kind. Each in vitro and in vivo experiments have indicated the presence of micro organism within the tumor-associated microbiota, which play an essential position in most cancers growth, immunosurveillance, metastasis, and chemoresistance. Molecular evaluation and bioimaging information have additionally proven the existence of intratumoral microbiota throughout main most cancers sorts.
There’s a lack of proof concerning the particular identification of host cells by means of which tumor-associated microbes work together with most cancers sufferers’ tumor cells. Moreover, little proof has been documented associated to figuring out particular cells that harbor organisms. The impact of exact host–microbial mobile interactions and spatial distribution of the intratumoral microbiota on their useful capabilities inside TME shouldn’t be obvious.
In regards to the Examine
16S rRNA gene sequencing of tumor tissues of CRC sufferers indicated the presence of assorted micro organism, together with Fusobacterium. The abundance of this micro organism differed between CRC sufferers. Dendrogram evaluation and principal part evaluation with beta range clustering indicated that almost all of most cancers sufferers had comparatively steady microbiome compositions. Nonetheless, most sufferers exhibited various levels of heterogeneity within the intratumoral microbiome composition.
The RNAscope–fluorescence in situ hybridization (RNAscope-FISH) imaging confirmed the heterogeneous spatial distribution of bacterial communities in TME. RNAscope-FISH-based information confirmed the presence of Fusobacterium nucleatum, which was additional validated by means of microbiome evaluation and quantitative PCR approach.
10x Visium spatial transcriptomics was additionally used to detect and analyze the spatial distribution of intratumoral microbiota of CRC and OSCC specimens. This strategy recognized 28% of captured spots inside OSCC tumors and 46% of CRC tumors.
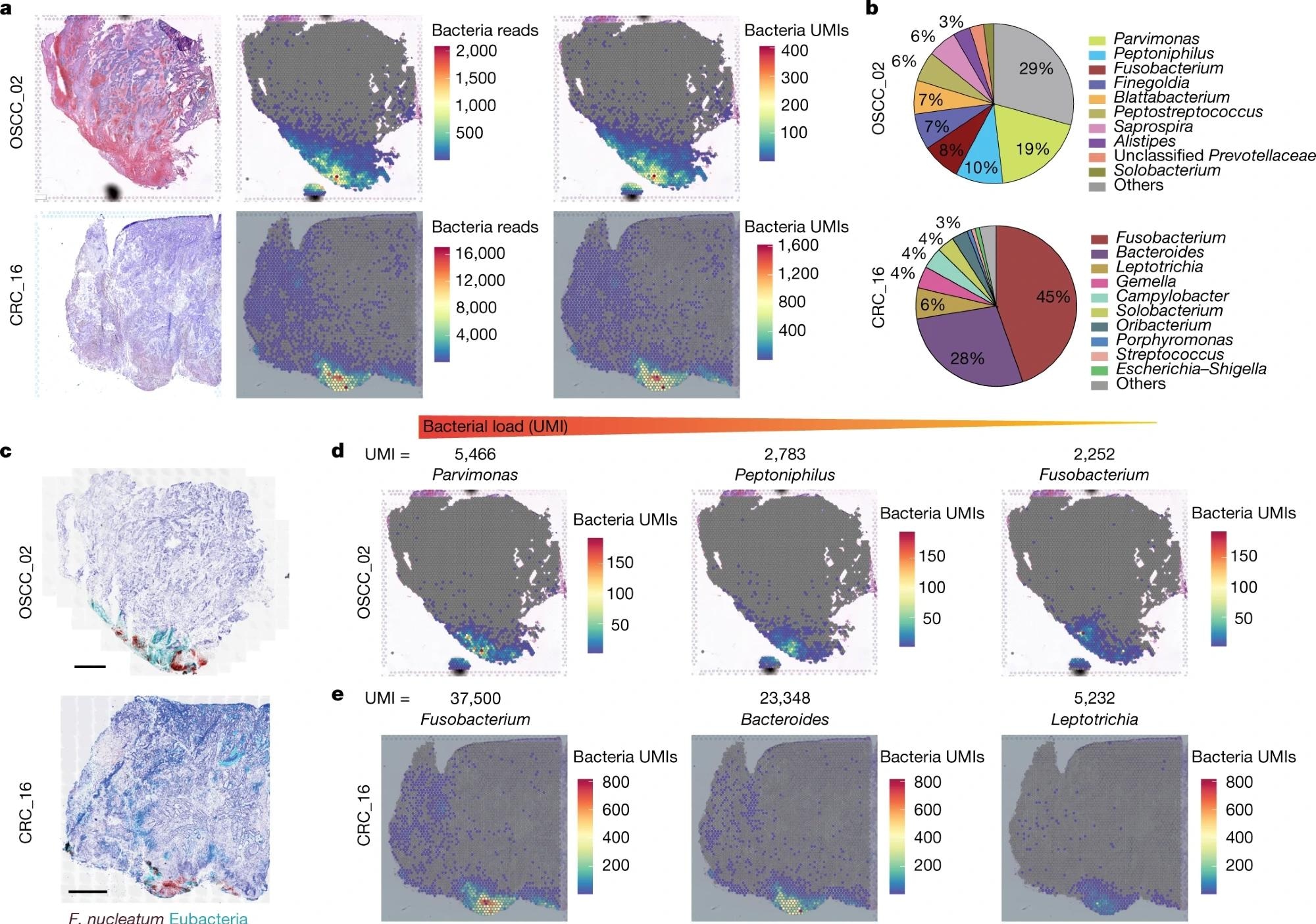 a, Haematoxylin and eosin (H&E) staining (left), spatial distribution of whole bacterial reads (centre) and whole UMI transcripts (proper) all through the tumor tissue within the 10x Visium seize slides from human OSCC and CRC specimens. b, Pie chart of the highest 10 most dominant bacterial genera detected within the 10x Visium RNA sequencing information from the OSCC and CRC tumors. c, RNAscope-FISH imaging displaying the distribution of micro organism throughout the tumor tissue in a sequential slide following the 10x Visium part. The F. nucleatum probe is crimson and the eubacterial probe is cyan. Scale bars, 1mm. d, Spatial distribution of Parvimonas, Peptoniphilus and Fusobacterium UMIs detected within the 10x Visium OSCC specimen information. e, Spatial distribution of Fusobacterium, Bacteroides and Leptotrichia UMIs detected within the 10x Visium CRC specimen information.
a, Haematoxylin and eosin (H&E) staining (left), spatial distribution of whole bacterial reads (centre) and whole UMI transcripts (proper) all through the tumor tissue within the 10x Visium seize slides from human OSCC and CRC specimens. b, Pie chart of the highest 10 most dominant bacterial genera detected within the 10x Visium RNA sequencing information from the OSCC and CRC tumors. c, RNAscope-FISH imaging displaying the distribution of micro organism throughout the tumor tissue in a sequential slide following the 10x Visium part. The F. nucleatum probe is crimson and the eubacterial probe is cyan. Scale bars, 1mm. d, Spatial distribution of Parvimonas, Peptoniphilus and Fusobacterium UMIs detected within the 10x Visium OSCC specimen information. e, Spatial distribution of Fusobacterium, Bacteroides and Leptotrichia UMIs detected within the 10x Visium CRC specimen information.
Within the OSCC tumor, Parvimonas, Peptoniphilus, and Fusobacterium have been discovered to be the dominant strains, whereas Fusobacterium and Bacteroides have been dominant genera within the CRC tumor.
10x Visium spatial transcriptomics approach enabled direct detection, quantification, and spatially mapping of viable micro organism inside most cancers sufferers’ intact tumor tissues. It additional indicated the complexity of intratumoral microbiota interactions throughout tumor tissues.
The GeoMx digital spatial profiling (DSP) platform helped quantify the expression profile of 77 proteins linked with most cancers development and anti-tumor immunity. This system, mixed with RNAscope and the immunohistochemistry (IHC) strategy, indicated that bacterial communities populate extremely immunosuppressive microniches and are usually not a lot vascularized. Moreover, bacterial strains are susceptible to inhabit malignant cells with decreased ranges of Ki-67.
The INVADEseq (invasion-adhesion-directed expression sequencing) approach was developed to evaluate bacterial-host cell-to-cell interplay throughout the TME and the impact on host cell transcriptomics. This system is related to the introduction of a primer to focus on conserved areas of bacterial 16S rRNA. Moreover, cDNA libraries with bacterial transcripts from the bacteria-associated human cells may be produced. One of many essential features of this methodology is that the introduction of primer doesn’t have an effect on the gene expression profile of human CRC cells.
The INVADEseq approach was validated utilizing the human CRC cell line HCT116 that was contaminated with bacterial species, particularly, F. nucleatum, Porphyromonas gingivalis, and Prevotella intermedia. It enabled the mapping of bacterial populations into single human cells. Importantly, INVADEseq confirmed the position of F. nucleatum and P. gingivalis in affecting most cancers cell heterogeneity. These bacterial strains alter distinct transcriptional packages that help in particular cell clustering. Alteration of transcriptional pathways can also be related to the manifestation of irritation, cell dormancy, metastasis, and DNA restore.
conclusions
The current research revealed that most cancers cells contaminated with micro organism have an effect on their surrounding as a single cell, which subsequently employs myeloid cells to the bacterial territory. Notably, the microbiome throughout the tumor was discovered to not be a random phenomenon. As a substitute, it was acknowledged that the presence of micro organism inside a tumor is a extremely organized course of in microniches with immune and epithelial cell features that affect most cancers development.
Although the present research targeted on two varieties of most cancers, the instruments and strategies may very well be used to check all main varieties of most cancers and people containing intratumoral microbiota.

